Emily Lakdawalla • Nov 30, 2012
More than you probably wanted to know about Curiosity's SAM instrument
So there has been a lot of hoopla about some early results from Curiosity's Sample Analysis at Mars (SAM) instrument this week. NASA has now issued a statement to the effect that these early results contain no definitive evidence of organics on Mars, and that there will be nothing to announce on Monday more exciting than the fact that the most expensive, most complex instrument ever sent to the surface of Mars is returning great-looking data for the first time. While I think that's exciting news, I'm sympathetic to the plight of a member of the general public who was misled by irresponsible speculation -- and not NASA's, by the way! -- into thinking that NASA discovered life on Mars. In an effort to bring some actual content to this discussion, I'm hereby offering a detailed explainer on how SAM actually works and what it is designed to detect. Settle in; it's 4000 words long.
Before I begin, though, I want to talk about a problematic word: "organic." Look up definitions of "organic" on the Web and a great many of them circle around to life. But when a space scientist talks about organic compounds, usually all they mean is a compound that contains carbon and hydrogen. So carbon dioxide, diamond, and cyanides are not organic, but hydrocarbons (methane, ethane, propane, etc...) and alcohols and amino acids are. The Wikipedia entry on organic chemistry discusses the problematic, occasionally arbitrary, and often not-very-useful nature of this hair-splitting. So I'm going to use a different phrase, "carbon-containing compounds," because that's really what we're talking about here.
SAM is described in a paper that was published in Space Science Reviews in April. The paper is open access (distributed under a Creative Commons Attribution license), so you can go read it if you want. The paper has 85 authors, so I'm going to refer to the writers as "the SAM team." SAM's principal investigator is Paul Mahaffy, its deputy principal investigator Pamela Conrad. I actually had a long conversation with Pan a couple of weeks ago, which you might enjoy:
Cosmoquest Science Hour, November 7, 2012: Curiosity update with SAM deputy principal investigator Pamela Conrad Enjoy an hour of explanation of Curiosity's Sample Analysis at Mars instrument with Emily Lakdawalla and Pamela Conrad.
Science Goals
The goals of the SAM investigation are to investigate three questions:
- What does the inventory of carbon compounds, or lack thereof, near the surface of Mars reveal about its potential habitability?
- What are the chemical and isotopic states of the lighter elements in the rocks, soils, and atmosphere and what do these reveal about potential habitability?
- How were past environmental conditions different from today's?
Just from reading these questions, you can get a pretty good idea of SAM's capabilities. It can detect carbon compounds in Mars rock and soil samples, if they exist, and can tell us what compounds they are. It can measure isotopic ratios of light elements. The compounds it finds can tell us about the environmental conditions that prevailed when they form.
Why are we asking these specific questions about Mars? It's more or less accepted that Mars' presently not-very-habitable environment wasn't always that way. Today it's cold, dry, and exposed to ionizing radiation. In the past, a magnetic field, thicker atmosphere, and warmer temperatures may have fostered the presence of liquid water sheltered from ionizing radiation. Even if the past climate wasn't nicer, past Mars definitely did have more liquid water, at least briefly or episodically, than Mars does now.
Life originated on Earth at or before 3.8 billion years ago. At the same time, it's possible life originated on Mars too, or maybe it originated on one world and was carried to the other. This possible Mars life is not obvious today, so if it ever existed, it either went extinct or underground. Since Mars hasn't been too geologically active since then, it's possible that evidence for this possible life that may have thrived in this possibly better ancient environment might be preserved in rocks exposed at the surface.
Interestingly, the odds of preservation of such ancient evidence for life are higher for Mars than for Earth because of Mars' substantially lower rate of geological activity. Most of Earth's most ancient rocks are destroyed or massively altered. Mars still has very, very ancient rocks exposed at the surface, very little changed from when they formed. Curiosity has been sent to a landing site exposing more ancient rocks than any lander or rover has ever studied. Unfortunately, even if any organic remains of any ancient Mars organisms were preserved in rocks, anything close enough to the surface to be accessible to Curiosity will have been attacked by radiation or oxidizing chemicals. Evidence of the original compounds will be in the form of different compounds, made from the breakup of more complex ones, that might possibly bear isotopic signatures that indicate a biogenic origin.
If you look back at the last two paragraphs, you'll see words like "possible" and "may" showing up quite a lot. The fact is, we know very little about Mars' ancient past, so it's really hard to predict what we'll find, and if it can tell us much of anything about whether or not ancient life existed. We will learn what we can, by exploring rocks ancient enough to preserve a record of what was happening way back then. Curiosity's mission goals are designed so that the mission will succeed at teaching us a great deal about Mars even if the rover finds zero evidence for ancient life.
Even if SAM does find carbon compounds, they are not likely to have been created by life. Meteorites called carbonaceous chondrites contain a lot of carbon, in chemicals like kerogens, amino acids, carboxylic acids, and nucleobases. A million kilograms of this material rains down on Mars every year. So near-surface carbon compounds are most likely from meteorites. Organics stuck inside rocks might be from ancient meteorites, or from Mars life.
The conservative assumption is that all the carbon is meteoric. No one will be able to make a claim for evidence of past Mars life without first eliminating the possibility that the carbon compounds came from meteorites. Since it's hard to prove a negative, there will not be any slam-dunk finding from SAM that says "Mars life was here."
There will have to be a story pieced together using multiple lines of evidence over a lengthy period of investigation. What the SAM inventory of organic compounds will really tell us is what raw materials would have been present when life was first getting started, either on Earth or on Mars.
Physical Description
This is SAM.
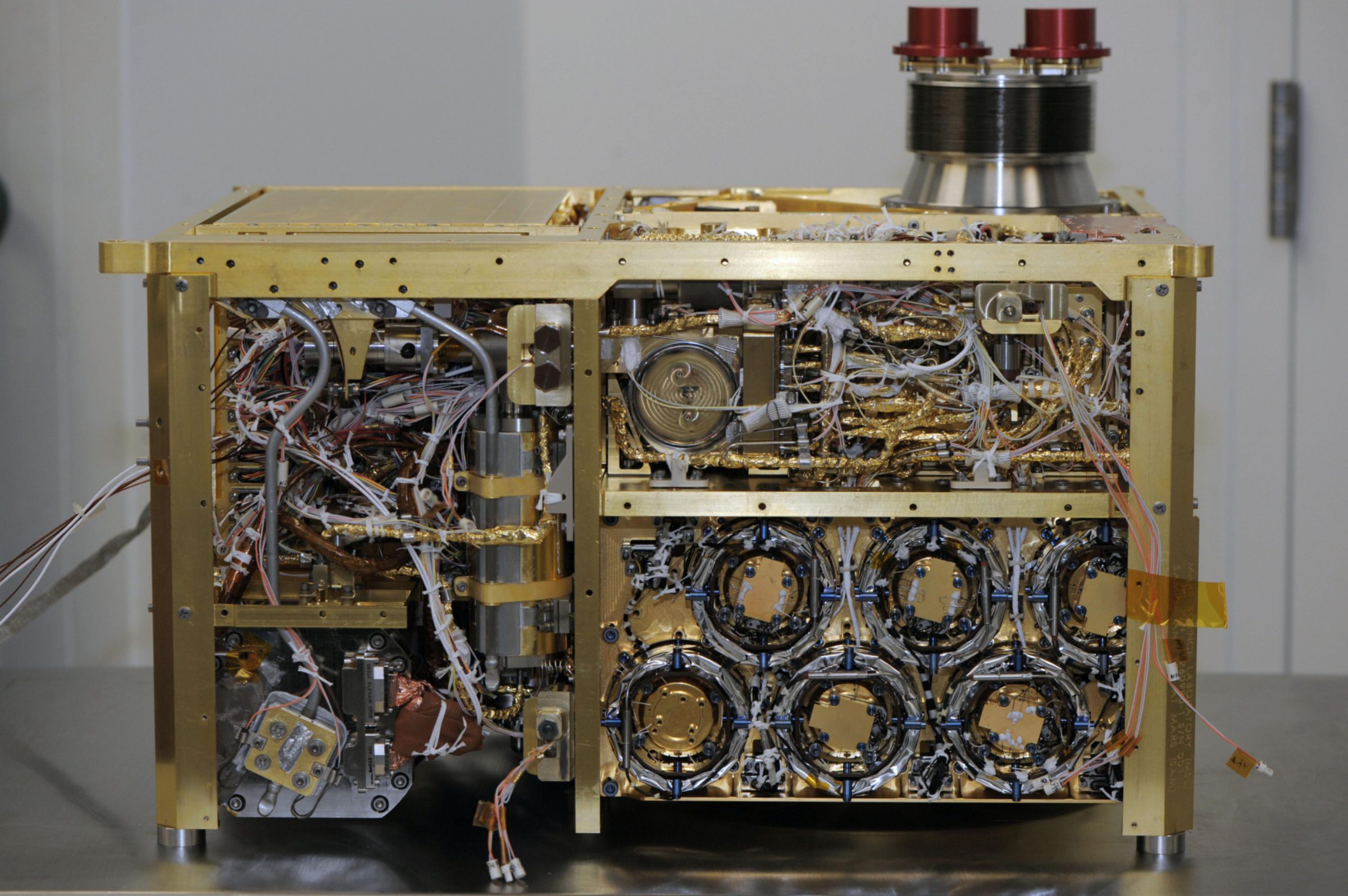
This is SAM being installed inside Curiosity, which had been rotated upside down for instrument installation. This is a really big instrument, weighing in at 40 kilograms, and is in large part responsible for Curiosity's enormous size.

On the outside, on the rover deck, SAM has two large inlet ports for solid samples. Curiosity uses its soil scoop or drill and a bunch of sieves and shakers in the turret on the robotic arm to prepare very small portions of finely powdery material to the ports. To learn about that part of the process, you should read the excellent series of guest blogs by Dan Limonadi, beginning with this one.

On Curiosity's starboard side, SAM has two smaller inlet ports for atmospheric gas intake.

SAM is really, really complicated, with lots of parts. Ultimately, all of its parts are designed to move gases to and through three main analytical instruments. Even when SAM takes in solid samples, what it measures are gases made from those solid samples by a variety of different methods.
Here's a list of the instruments and parts and what they do. I'm also writing down the acronyms and initialisms that you'll hear the science team use to name them, but the only acronyms I sometimes use are the ones that actually name instruments. "SAM" is the name of the whole package, and its three analytical sub-instruments are the gas chromatograph (GC), the quadrupole mass spectrometer (QMS), and the tunable laser spectrometer (TLS).
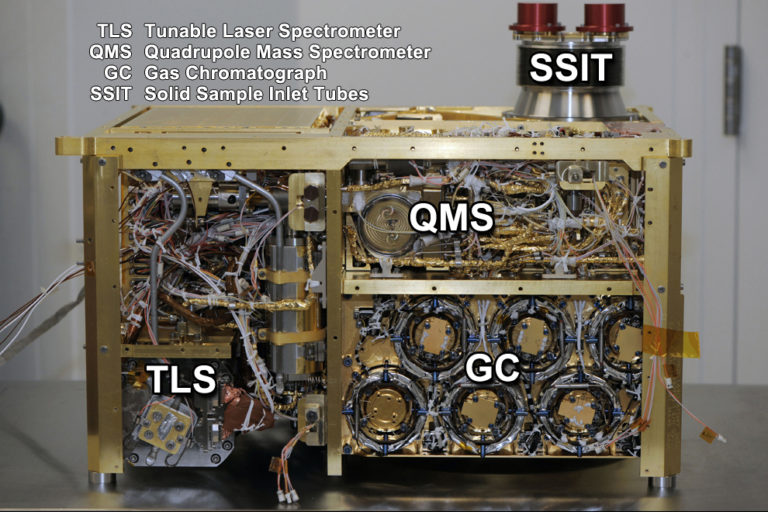
Instruments
Quadrupole Mass Spectrometer (QMS): A mass spectrometer uses electromagnetic fields to separate charged particles according to their mass-to-charge ratio (m/z). Curiosity's mass spectrometer is sensitive to molecular masses from 1.5 to 535.5 Daltons, with a resolution of 0.1 Dalton. That's a pretty high mass for a spaceborne mass spectrometer, as far as I know. The Cassini Saturn orbiter also has a quadrupole mass spectrometer (its Ion and Neutral Mass Spectrometer or INMS), but that one only reaches to 99 Daltons. The higher number is needed for Curiosity because of the larger carbon compounds that they hope to detect. The quadrupole design on Curiosity's instrument is very similar to the one that was on the Galileo Jupiter atmospheric probe. Curiosity's mass spectrometer has an ion source and detector assembly based on one developed for the ill-fated comet mission CONTOUR. It is more than 10 times more sensitive than Cassini's. The QMS can operate continuously, taking readings every 0.02 seconds.
Gas Chromatograph (GC): The gas chromatograph allows Curiosity to separate a mixture of gases, which improves the mass spectrometer's ability to identify them. Gas chromatography helps a mass spectrometer distinguish among different organic compounds that have the same molecular weights. It is particularly focused on compounds made of light elements: hydrocarbons and atmospheric gases. There are actually six different gas chromatograph "columns." Each of the six columns is a tube 30 meters long but only a quarter of a millimeter in diameter. The tubes are wound into coils to pack them inside the instrument, so each of the "columns" actually looks like a disk, from the outside. Thus the six circles making up the GC instrument in the photo of SAM. Each of the columns is designed to work on different (but overlapping) ranges of molecular weights. One column targets small carbon molecules (chains of 1 to 4 carbon atoms) and ammonia, two look at medium molecular weight hydrocarbons (5- to 15-carbon chains), and so on. Three of the six columns have traps that adsorb interesting gas species as they arrive at the start of the column, in order to concentrate them; flash heating of the traps releases the gas molecules all at once, boosting the chromatograph's ability to separate them into different constituents.
The inside surface of each column is coated with a different substance. The substances grab hold of gas molecules and release them. Different gas molecules have more or less affinity for each column's coating. So the long, thin tube separates different gas molecules by their affinities -- in general, the less sticky molecules exit the column first, the stickiest last. In addition, large molecules tend to move more slowly down the column than small molecules. As gas molecules exit the tube, they pass across a thermal conductivity detector (TCD), which can detect the major species in the gas down to the part-per-million level. From there, the separated gases go into the mass spectrometer. This is not the first gas chromatograph mass spectrometer (GCMS) sent to Mars, but it's the first one since the Viking landers. The design of Curiosity's is based upon the GCMS that was on Huygens, the Titan atmospheric probe.
Tunable Laser Spectrometer (TLS): The tunable laser spectrometer is different from the gas chromatograph and quadrupole mass spectrometer. The GC and QMS are designed to survey across a wide range of atomic masses, measuring the abundances and isotopic ratios of a wide variety of gases. The tunable laser spectrometer measures only three specific gases: methane, carbon dioxide, and water. It can make very precise measurements of the isotopic ratios of hydrogen, carbon, and oxygen that they contain by looking at specific-wavelength emission lines of the different isotopes of those three elements. The tunable laser spectrometer doesn't operate continuously, unlike the QMS. SAM waits until the TLS chamber is filled to some desired pressure, and then performs a TLS reading. Then it usually will pump out some of the gas and perform another reading at a lower pressure. It usually repeats this a few times. Reading at multiple different pressures makes sure that there will be at least one reading for each gas at which the detector is not saturated.
Used in concert with the GC and QMS, the tunable laser spectrometer can help determine the abundances of gases that can be hard to pull out of mass spectrometer measurements because of ambiguous molecular masses. These are things like carbon monoxide/molecular nitrogen (which both have a mass around 28 Daltons), nitrous oxide/carbon dioxide (44 Daltons), or deuterated methane/heavy-carbon methane (17 Daltons).
How It All Works:
The guts of SAM are fiendishly complex, a spaghetti of tubing and valves and manifolds and heaters and pumps and gas reservoirs. ("Manifolds" are chambers from which there are many openings -- kind of like junction boxes but for gas.) SAM uses a supply of helium, carried with it to Mars in two reservoirs, to direct tiny puffs of gas and powdered rock from inlets to sample cups and ovens and gas traps and science instruments. The pumps establish pressure differences in different manifolds that act to pull gases along the tubes. Here is a diagram:
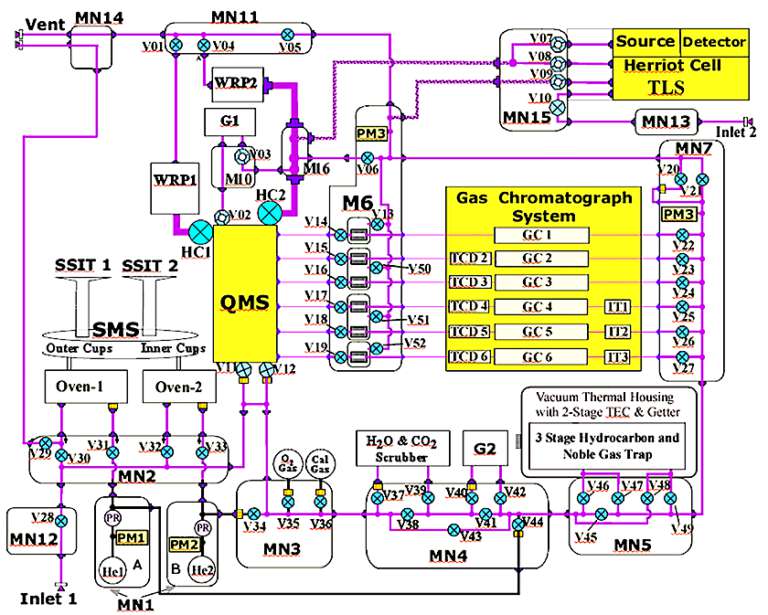
The various valves, manifolds, and traps give the SAM team tremendous control over the movement and processing of air and rock powder samples. To reduce this complexity to something more manageable, they have designed a short list of typical experiments, which can be modified using a scripting language developed especially for SAM. Here are those most typical experiments.
Experiments Performed on Atmospheric Samples
To perform any atmospheric analysis, SAM begins by activating its pumps and performing background readings from QMS and TLS. Then SAM heats the manifolds and tubes that it will use to transfer the gas from outside the rover to the instruments. (A typical temperature is 135°C.) Once the instrument is at temperature, SAM opens a valve to take in air.
Direct analysis of atmospheric gas: This will be the most frequently performed atmospheric sample experiment, involving mass spectrometry and laser spectrometry measurements of the atmosphere. SAM performs a QMS measurement first, then it uses the TLS. The TLS measurement is performed several times with the pumps establishing different pressures inside the instrument.
Noble gas analysis: SAM directs air into manifolds containing "scrubbers" and "getters." These can bind the most abundant constituents of the atmosphere -- carbon dioxide, nitrogen, and water -- leaving behind the trace constituents, including noble gases (and also methane). Argon and neon are the most abundant noble gases in Mars' atmosphere, so they comprise the bulk of the remaining gas. This remaining gas can be directed to the QMS for isotopic analysis. Or it can be sent to another manifold, where a thermoelectric cooler traps the heavier noble gases xenon and krypton. After SAM pumps out the (mostly argon and neon) remaining gas, it warms the cold trap to release the xenon and krypton to be pumped to the QMS, again for isotopic analysis.
Methane analysis: In a procedure similar to the noble gas analysis, SAM uses the getters and scrubbers to remove carbon dioxide and nitrogen from the air. The remaining gas (which is mostly argon, but which will contain some of the methane) is directed to the TLS. SAM repeats this procedure multiple times to raise the density of methane gas in the TLS -- if it's there at all.
Atmospheric enrichment: Sort of the opposite of the methane analysis, SAM directs atmospheric gas over the scrubber, then pumps out the remainder and releases the trapped gases. The scrubber's main purpose is to take up carbon dioxide and water, but it also traps hydrocarbons heavier than methane as well as other trace gases. So whatever has been taken up by the scrubber is released either to the TLS -- which can then perform an isotopic analysis of the water -- or it is sent to another manifold containing a trap that traps hydrocarbons, which can later be released to the QMS or GC.
Experiments Performed on Solid Samples
SAM processes solid samples with its Sample Manipulation System (SMS). The heart of the Sample Manipulation System is a carousel containing 74 sample cups in two concentric rings. There are three main kinds of cups: 59 quartz cups, 9 wet chemistry cups, and 6 calibration cups. The carousel can deliver individual cups to one of two ovens by rotating to place the chosen cup underneath the oven, then disengaging the cup from the carousel and lifting the cup upward to put the sample cup inside the oven. It shoves upward with force powerful enough to create a tight seal between the top of the sample cup and the roof of the oven. One of the ovens has a maximum temperature of 950°C, the other 1100°C. Baking to these high temperatures requires a huge (for Curiosity) amount of energy; on any sol that SAM's ovens are on, Curiosity can do almost nothing else.
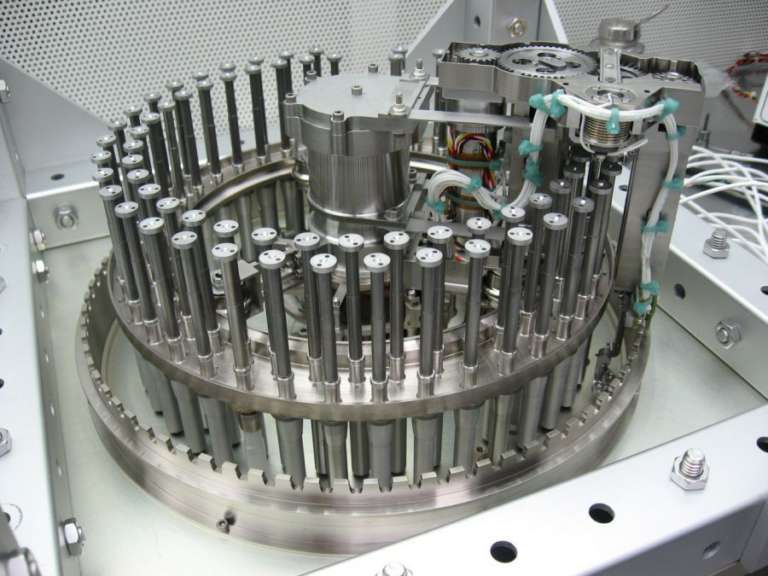
Curiosity uses the tools on the end of the robotic arm to obtain some soil or rock powder and sieve a sample so that all particles are smaller than 150 microns in diameter. Then the arm portions out a very small amount (0.078 cubic centimeters -- think of a round pill a centimeter across and a millimeter thick) and delivers it to SAM through one of the two the Solid Sample Inlet Tubes (SSITs). These tubes vibrate in order to make sure that the sample doesn't get stuck on its way in. The sample drops into a waiting cup.
Evolved gas analysis: This is the most common solid sample measurement. Of SAM's 74 sample cups, 59 are devoted to evolved gas analysis. SAM prepares for this experiment with a little housekeeping, cleaning a cup. Cup cleaning involves placing a cup inside an oven and baking it above 900°C while sending occasional puffs of helium inside. The cup stays inside the oven, nice and clean, until the next sol, when SAM takes it out and rotates it underneath the inlet. The inlet opens, the arm funnels a sample inside, and SAM puts the cup back in the oven.
Just as for atmospheric analysis, SAM starts by heating all the manifolds and tubing that will be carrying gases, then takes background measurements with the QMS and TLS. SAM warms the oven to 125°C and holds that temperature for 10 minutes. A constant flow of helium brings gases from the oven to the QMS. The oven starts ramping up the temperature, and the QMS continuously scans. The composition of the gases coming out of the oven will change as the temperature rises; the most volatile species will wind up in the QMS first, the most refractory last. Some of the stuff in the sample goes directly from solid to gas -- its abundance in the QMS was its abundance in the solid sample. But most of the materials decompose into simpler compounds as the temperature ramps up; the SAM team has to work backward from the simpler gases that QMS measures to the compounds that decomposed to make them. The temperature at which each gas appears in the QMS can be diagnostic of the material that decomposed. It's a lot like reading infrared spectra, where there are some materials whose presence will be obvious in the QMS data, and others that can be ambiguous and will have to be teased out with careful analytical and modeling work.

There are ways that the evolved gas analysis can be tweaked. At certain times, some of the evolved gas can be directed into the TLS (where it will be held for later analysis of water, carbon dioxide, and methane abundance and isotopes), or it can be sent over the hydrocarbon trap to, well, trap hydrocarbons.
Gas chromatograph mass spectrometer analysis: After an evolved gas analysis, SAM will usually go on to perform gas chromatography and mass spectroscopy of the stuff that was caught in the hydrocarbon trap. SAM heats its hydrocarbon trap, which releases the stuff that stuck to it. It sends the released hydrocarbon gases through one of the six gas chromatograph columns. As the gas exits the column, it's measured with the thermal conductivity detector and then with the mass spectrometer.
Tunable laser spectrometer analysis: Once the gas chromatograph analysis is done, SAM will usually go on to use the tunable laser spectrometer to look at abundances of and isotopes in water, methane, and carbon dioxide.
The whole sequence of evolved gas analysis - gas chromatograph mass spectroscopy - tunable laser spectrometry takes 4 to 6 hours to complete and most (if not all) of the battery life Curiosity has available in any given sol. If there's not enough power available to do all three analyses in one sol, the activities can be spaced out over multiple sols.

Solid sample combustion: After evolved gas analysis, there will almost always be a residue left inside the sample cup. Some carbon compounds may not become volatile even at the highest temperatures SAM's ovens can achieve. To try to get at the composition and isotopic ratios in this stuff, SAM can actually combust it. SAM contains a reservoir of oxygen. With oxygen puffed into the oven, heating the cup above 750°C for some period turns refractory carbon compounds into (primarily) carbon dioxide. By sending this gas to the TLS, SAM can determine the isotopic ratio of ordinary carbon-12 to heavy carbon-13 in the material.
With evolved gas analysis or combustion finished, there will be still be a residue left in the cup. This material will remain in the cup for the rest of the mission. But since it is, by definition, material that wouldn't exit the cup even when heated to the maximum temperature, SAM can actually reuse the sample cup for another, later analysis, and the residue shouldn't contaminate the results. The cups are deep enough to receive many samples.
Wet chemical analysis: Some of the most interesting carbon compounds that Curiosity might find are ones that SAM can't pick up through any of the above methods. These are large, astrobiologically interesting compounds like amino acids and carboxylic acids. They aren't volatile at low temperatures, and they rapidly decompose to smaller compounds at higher temperatures, so they're essentially invisible. To test for these, SAM's carousel carries nine precious wet chemistry cups, sealed with foil caps, containing solvents that break large hydrocarbons into smaller ones that SAM's gas chromatograph can separate. Each wet chemistry cup can only be used once. SAM pierces a cup, receives a sample, and puts the cup into an oven to be incubated at a relatively low temperature. The gases that come off the wet chemistry cup are analyzed using gas chromatography and mass spectroscopy.
What Will the SAM Data Set Be?
SAM is required to obtain specific data sets to specific performance levels.
- Inventory volatile organic compounds in rocks and soils, measuring anything that's more abundant than a few parts per billion (by mass).
- Measure the distribution of molecular weights and chemical structures for organic compounds in rocks and soils. Wet chemistry analysis will be required to get at some of these. SAM is required to inventory compounds containing everything up to 20 carbons.
- Inventory certain specific astrobiologically interesting molecules including amino acids, amines, and carboxylic acids.
- Measure the carbon-13/carbon-12 ratio in refractory carbon compounds. This is where the combustion analysis comes in. SAM is required to measure abundances of such refractory compounds to the part-per-million level.
- Measure the distribution of oxidation states of organic compounds. This gets at the environmental conditions that prevailed when they formed.
- Inventory and temperature-profile volatile inorganic compounds in rocks and soils. This will get at abundances of certain minerals present in the rocks, like carbonates, sulfates, and clays, to the part-per-million level.
- Measure the abundance and carbon-13/carbon-12 ratio of methane.
- Measure how atmospheric gas concentrations vary over the course of a sol and over the course of Mars' seasons.
- Measure the relative abundances and isotopic ratios of the noble gases argon, neon, xenon, and krypton. There are specific precision levels required for all these abundances and ratios; if you care about those specifics, look up Table 5 in the paper.
There you go, 4000 words on SAM. I hope this will be helpful to some of you in understanding what we can and can't learn from the work done by this amazing instrument!
Thanks to Sarah Horst for helping me explain some principles of gas chromatography.
Let’s Go Beyond The Horizon
Every success in space exploration is the result of the community of space enthusiasts, like you, who believe it is important. You can help usher in the next great era of space exploration with your gift today.
Donate Today

 Explore Worlds
Explore Worlds Find Life
Find Life Defend Earth
Defend Earth

