Emily Lakdawalla • Oct 12, 2012
First science reports from Curiosity's APXS and ChemCam: Petrology on Jake Matijevic
Members of the media have been hassling the Curiosity science team for weeks to report some initial results from the use of the ChemCam and APXS to analyze rock compositions. Today, they finally got what they asked for -- but unless you've taken a class in igneous petrology, you'd be hard pressed to explain what those results were, much less what they mean! So that means it's up to Kelly Beatty and me to explain what the heck the geologists were talking about today. Before I get into technical details, let me just remind you where we are: we are here, in this stunning landscape of eroded layered bedrock.

Let's begin at the beginning, with an explanation of what a rock is. No, I'm not being patronizing. "Rock" is one of those dangerous words in science that means something somewhat different to geologists than it does to non-geologists. The word "rock" is actually defined in the glossary of my battered copy of Press & Siever's introductory geology textbook: "A solid, cohesive aggregate of grains of one or more minerals." Mineral is italicized because it, too, has a precise definition: "A naturally occurring, solid, inorganic element or compound, with a definite composition or range of compositions, usually possessing a regular internal crystalline structure." They don't define "composition," so I will: the composition is an expression of the ratio of different chemical elements that make up a mineral.
So: you have elements in certain abundances. Those elements are combined into minerals with specific compositions. A rock is an assemblage of grains of one or, usually, more minerals. Sometimes, when a geologist uses the word "rock," they don't just mean a thing you can hold in your hand; they mean the entire rock unit, a formation that can go on for miles. Here's a rock from Earth, a basalt, in which you can see individual mineral grains:

To a geologist, an individual rock (or rock formation) is a record of the series of events that caused that rock to form. Lots of different kinds of rocks can have the same bulk composition. Which minerals get made from the available elements depends on things like the ambient temperature and pressure when the minerals formed. If you have a rock that contains minerals that required high temperature and pressure to form, then you have the outline of in interesting story: you know that that rock originally formed at some depth below the surface, and then some geologic process brought it to the surface and exposed it. How the mineral grains are held together is another important clue to the story. If they're interlocking crystals, your rock is likely igneous (formed from a melt). If they're rounded grains held together by some kind of cement, your rock is likely sedimentary.
Most of Curiosity's instruments and tools are designed to make the observations necessary to read the life stories of rocks. MAHLI -- the hand lens imager -- can see the structure of the grains (if they're not microscopic) and how they're held together. APXS and ChemCam both measure the elemental composition of the rock. They can't see minerals; they just see elements. It's SAM and CheMin, the analytical laboratory instruments in the belly of the rover, that will eventually be used to perform mineral analyses.
Today's results came from ChemCam and APXS, the elemental analyzers, in measurements they performed on the rock dubbed Jake Matijevic at their first science stop, beginning sol 43. The team selected the rock for their first in-situ analysis because it looked like a homogeneous basalt, a type of igneous rock common on all planetary bodies (think Hawaii, the lunar maria, the Martian meteorites…). But the chemistry of this particular basaltic rock surprised them. Here is Jake, in a useful set of images assembled by Damien Bouic:
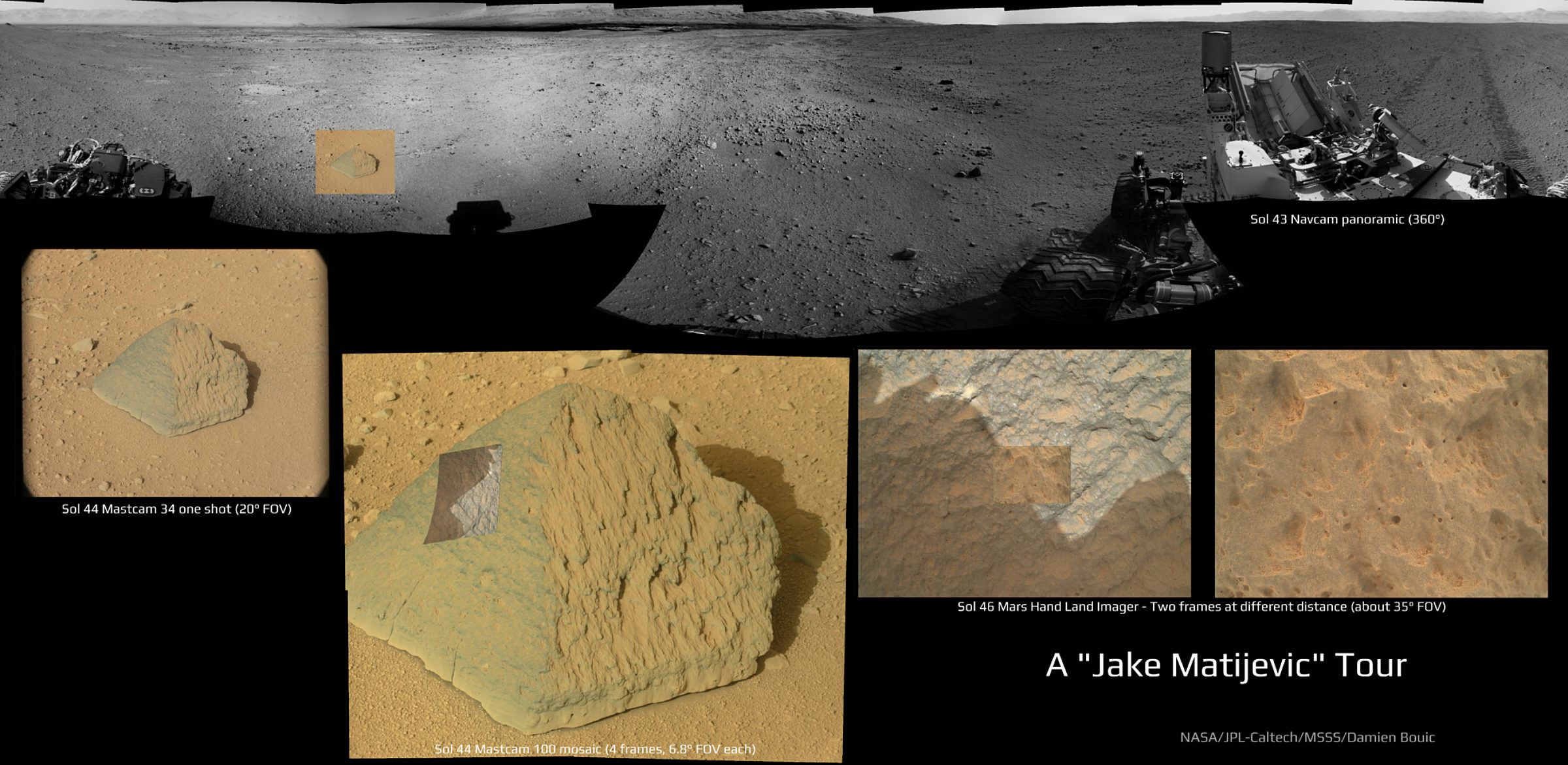
And here is a context image from today's briefing, showing where they performed analyses. The red dots are ChemCam shot points, and the purple circles are two spots measured by APXS.
Let's begin with APXS. Here's a picture of the instrument, from my post about the visual instrument checkout:
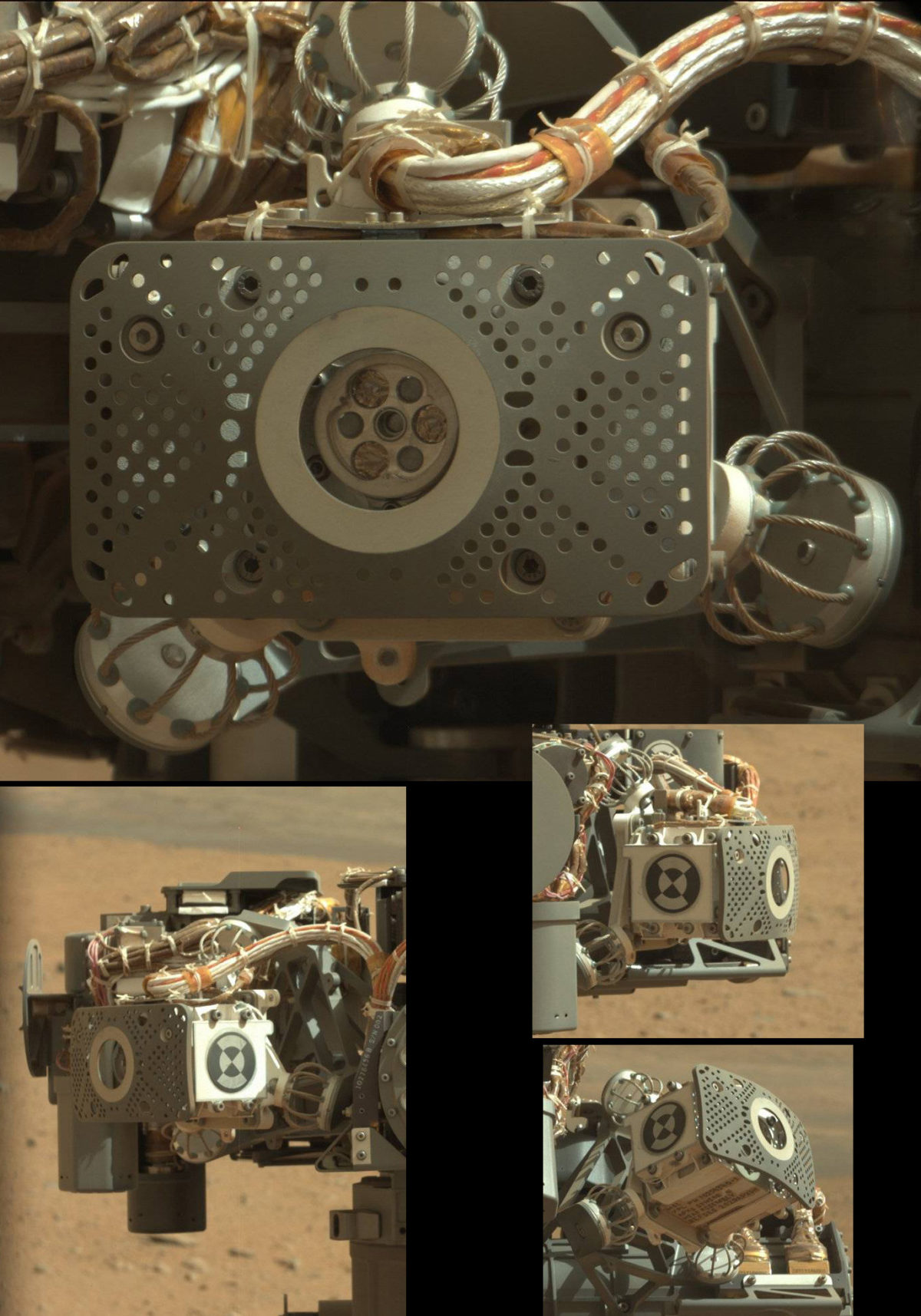
APXS measures chemistry from a larger single area than ChemCam, so it reads something closer to the bulk elemental composition of the rock. The APXS has a little lump of radioactive curium-244 that emits X-rays. When APXS is held close to a rock, the X-rays strike the atoms at the surface of the rock, which emit or fluoresce X-rays in response. Each chemical element emits X-rays with different, diagnostic energies. APXS detects these emitted X-rays, counting them and recording their energy. APXS benefits from long exposures: the longer it's held against a rock, the more X-rays it counts, and the more sensitive it is to trace elements in the rock. Curiosity's APXS improves on Spirit and Opportunity's by working much faster: it counts as many X-rays in one hour as Opportunity counts in 17 hours. As far as I understand it, this is simply because Curiosity's APXS can get closer to the rock than Opportunity's can!
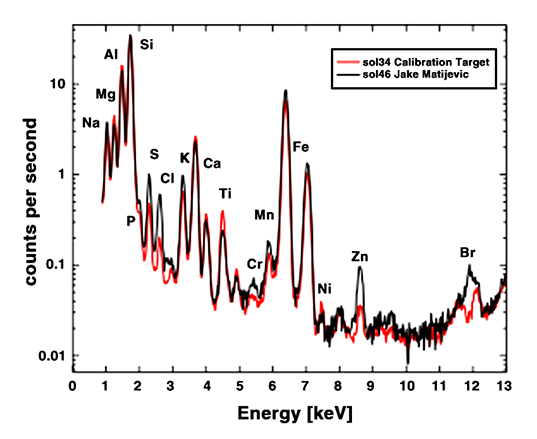
Here's the graph that Ralf Gellert, APXS principal investigator, showed today. This graph contains two squiggles. The black one is that measured on Jake Matijevic. The red one was measured on the calibration target, a bit of Earth basalt that Curiosity brought with it, whose composition is extremely well understood.
The graph is a bit confusing, because we're not really meant to compare the black and red lines. We're meant to compare the black line to one that's not even on the graph, representing the kinds of rocks that Spirit and Opportunity have seen, and which would have a squiggle that looks slightly different from both the black or the red one. According to Gellert, if you compare the APXS measurements on Jake to those made by Spirit and Opportunity on basalts at Gusev crater and Meridiani planum, you find that Jake "is low in magnesium and iron, [and] high in elements like sodium, aluminum, silicon and potassium, which often are in [alkali] feldspar minerals. It has very low nickel and zinc. The salt-forming elements sulfur, chlorine and bromine are likely in soil or dust grains visible on the surface of the rock."
To a geologist, that string of four elements -- sodium, aluminum, silicon, potassium -- immediately makes one think "feldspar," and a particular flavor of feldspar (rich in alkali elements sodium and potassium) that I don't know if we've ever seen on Mars before. It's the commonest rock-forming mineral on Earth, the pink or opaque white or sometimes gray material in granite countertops. That, combined with the information that the rock is relatively low in magnesium, iron, nickel, and zinc, told every geologist listening to the call that Jake is a rock that formed from a rock melt that evolved, changed, from a straightforward melted Mars rock. (Or, that it was a melted rock made of material that had gone through some such process.) It's a rock that formed through processes that we know in Earth geologic environments, but not one we've ever seen on Mars.
So let's talk about how rock melt compositions can evolve. But we'll begin with something more familiar (particularly to geologists!): liquor. There was a petrologist member of the science team on the panel today, Ed Stolper, who used this simile to try to explain the concept to the listening journalists. Specifically, Stolper talked about making applejack. Applejack is a liquor made by taking a hard cider (usually around 5% alcohol by volume) and storing it at a temperature below freezing. As the cider cools, water ice begins to crystallize. This ice is nearly pure H2O, water; alcohol has a lower freezing temperature, and the other stuff in the liquid that give the applejack its flavor stays in solution. Because water has gone in to the solid ice, the remaining liquid is relatively depleted in water, and relatively enriched in alcohol and other stuff. Take the ice out and throw it away, and you've begun concentrating the liquor, increasing its proportion in the liquid. The lower the temperature you take it to, the more water ice freezes out, and the more concentrated the stuff that's not water gets in the remaining solution. I checked last night, and the applejack in my liquor cabinet is 40% alcohol by volume.
This process is called fractional crystallization, and it also operates inside magma chambers. Start with a melted rock and then allow it to cool, and the first mineral that crystallizes out has a different composition than the bulk composition of the melt. What mineral crystallizes out first depends on the composition of the melt in super complicated ways that it takes textbooks and ongoing scientific careers to describe. But when you've got a melt with the composition of the mantle of Mars or Earth, what crystallizes out first are iron- and magnesium-rich minerals that have relatively low amounts of silica: olivine (Mg2SiO4 or Fe2SiO4) and then pyroxene (like CaMgSi2O6). If those initial crystals go away -- for instance, if they settle out -- what's left behind is a melt containing relatively less iron and magnesium and relatively more silicon, potassium, sodium....all the stuff they found in this rock.
When you take a geology class that's about Earth, you learn about how fractional crystallization can start with a basaltic melt (one that's rich in those dark minerals and iron and magnesium) and work (or evolve) your way up to increasingly silica-rich rocks, from basalt, to andesite, to rhyolite or granite. Jake is not a granite or even an andesite. We're still talking about a very dark, basaltic rock here. But it's a little up the spectrum from any other basalt we've seen before, to the point that it's called an alkali basalt.
APXS wasn't the first instrument to detect this feldspar-like composition in a rock. Roger Wiens, the principal investigator on ChemCam, divulged today that they had been seeing feldspar-like compositions on rocks they've been shooting their laser at since August. That was an "aha!" moment for me; it explained why we have been hearing so little from the ChemCam team, until now. The observation of so much feldspar is so unexpected that they had to be really thorough, checking that their instrument was functioning properly, corroborating its readings with measurements by APXS, and so on, before they were ready to come forward with it.
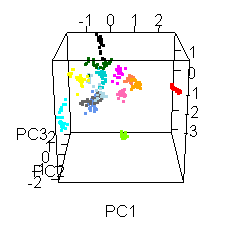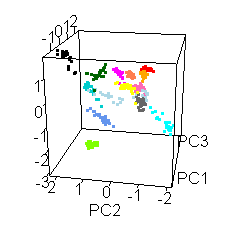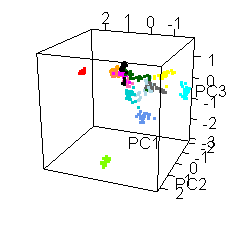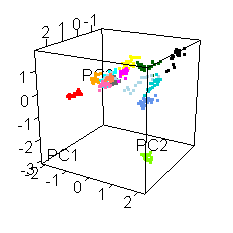
Wiens had an interesting extra wrinkle to the story. Go back up to the picture of Jake with all the shot points on it and you'll see they shot fourteen different locations. Each of those shot points actually represents 30 laser shots, each of which was analyzed by the ChemCam spectrometer.
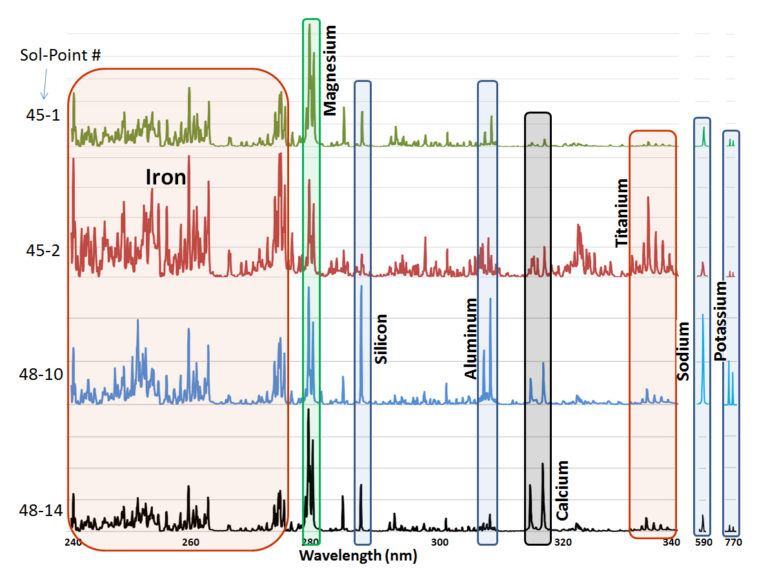
He shared this fun spinny cubic graph based on a principal components analysis of the data. Each of the fourteen shot points was assigned a different color. For each shot point, there are 26 dots for the laser shots (they throw out the first four because those are mostly sampling dust coating the rock). You're not meant to be able to read anything specific about the composition of the rock from this graph. The point is that every one of the fourteen shot points had a composition that was distinct from the other thirteen. Each color forms a tight cluster, and there's very little overlap. That's Jake's second surprise: it was a surprisingly heterogeneous rock on the scale of the ChemCam analyses. ChemCam is probably sampling individual crystals of different minerals.
Wiens shared a graphic that supports this statement: spectra from four of the shot points. These are really quite beautiful. The first, high in magnesium and (to a lesser extent) iron, is likely a grain of olivine (more commonly known as peridot, the birthstone of August; compositions range from the endmembers Mg2SiO4 to Fe2SiO4). The next one has a ton of titanium, so it could be a something like ilmenite (FeTiO3). The next one has a big spike for silicon, aluminum, sodium, and potassium: an alkali feldspar (compositions ranging from KAlSi3O8 to NaAlSi3O8). The bottom one has calcium and magnesium: likely a pyroxene (where the calcium and magnesium endmember has the composition CaMgSi2O6).
The next graphic he showed was super cool. What he's plotting here are elemental abundances of magnesium and calcium as measured in that pyroxene grain. With every shot, the abundance of magnesium and calcium increased, as they drilled down into the pyroxene grain.
All this compositional stuff is nifty, but we're not yet telling a story. The problem is, it's hard to tell a full story when all you've got is one rock. To learn more about what the composition of this rock means for the story of its origin, you really have to see how it fits in to the larger context of rocks in the area. It's just one rock, and it's not even in place; there's little context to help us understand how it formed, what the geologic environment was like. Stolper mentioned that the rock is a relatively uncommon type on Earth, generally found in ocean island volcanic settings (think Hawaii) or associated with eclogites (which are pretty unusual themselves). But just because that's where it found on Earth, doesn't mean that there was the same kind of environment on Mars. (And how exactly Earth generates melts with this composition is still a subject of debate.)
The journalists on today's teleconference pushed Stolper hard for statements about what this rock says about Mars' history, about the environment that prevailed when Jake formed. His answers likely dissatisfied them; they were equivocal and noncommittal. But that was appropriate. The news today is that in investigating a common rock type (basalt) to cross-calibrate two instruments, they found an uncommon composition, something unique, something that requires a dynamic, evolving geologic environment of an as-yet-unknown nature, something that opens up a new space of possibilities for types of igneous geology that can be imagined for Mars' past.
I turned to friendly petrologist and Curiosity team member Michelle Minitti to review this article (many thanks, Michelle!), and she explained that "the essence is that we found a new Martian basalt composition, one that can be compared and contrasted to the basalts we already know from Mars (the Martian meteorites and those measured by Pathfinder, Spirit and Opportunity) to improve our understanding of how Mars formed and changed through time. The chemistries of the basaltic Martian meteorites are importantly different from the basalts at Spirit, and both seem to be different in a meaningful way from Jake M. Each basalt data point provides a new window into what Mars is fundamentally made of, how it differentiated and evolved through time."
Mars' past is still mostly mysterious, and this is just one little clue. But it's a brand-new kind of clue we've never seen before, and that is something to be excited about!
Let’s Go Beyond The Horizon
Every success in space exploration is the result of the community of space enthusiasts, like you, who believe it is important. You can help usher in the next great era of space exploration with your gift today.
Donate Today

 Explore Worlds
Explore Worlds Find Life
Find Life Defend Earth
Defend Earth